h2so3|h2so3 state of matter : Manila Modify: 2024-06-22. Description. Sulfurous acid appears as a colorless liquid with a pungent burning sulfur odor. Corrosive to metals and tissue. CAMEO Chemicals. Sulfurous acid is a sulfur oxoacid. It is a conjugate . Usado Fácil - O maior e melhor portal de veículos de Mato G.
0 · how to name h2so3
1 · h2so3 type of compound
2 · h2so3 state of matter
3 · h2so3 compound name
4 · h2so3 chemical formula
5 · h2so3 basicity
6 · h2so3 aq name
7 · h2so3 acid name
8 · More
Resultado da As mais recentes transferências. Esta página mostra as últimas transferências confirmadas. Origem, destino, valor da transferência, valor de mercado e .
h2so3*******Sulphurous Acid is a weak diprotic acid and a tautomer of sulfonic acid. It is used in various industries, but it is also toxic and corrosive. .Modify: 2024-06-22. Description. Sulfurous acid appears as a colorless liquid with a pungent burning sulfur odor. Corrosive to metals and tissue. CAMEO Chemicals. Sulfurous acid is a sulfur oxoacid. It is a conjugate . In this video we'll write the correct name for H2SO3.To write the name for H2SO3 we’ll use the Periodic Table and follow some simple rules. Because H2SO3 has.Sulfurous acid is an unstable, weakly acidic compound that is produced when sulfur dioxide is dissolved in water. It is used in various industries, such as paper pulp, .
Learn about the sulfurous acid (H2SO3) compound, its molecular formula, structure, and properties. See how to draw the Lewis structure of H2SO3 and its formal .
Learn why sulfuric acid has a tetrahedral geometry and an $sp^3$ hybridization, despite having a 10-electron configuration. See explanations, diagrams, and references from experts and users on this .
In this video we'll write the correct name for H2SO3.To write the name for H2SO3 we’ll use the Periodic Table and follow some simple rules. Because H2SO3 has. The name of the acid is based on the anion attached to the hydrogen. 5.9: Naming Acids is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. An acid can be defined in several ways. The most straightforward definition is: an acid is a molecular compound that contains one or more .
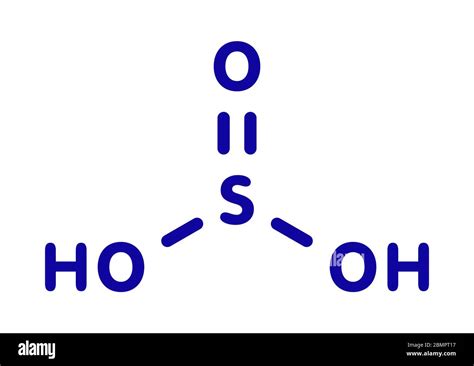
Finally, add together the total mass of each element to get the molar mass of H2SO3: 2.01588 g/mol + 32.065 g/mol + 47.9982 g/mol = 82.07908 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in H2SO3, divide each total from step 3 by the total molar mass found in step 4: As concluded in How relevant are S=O and P=O Double Bonds for the Description of the Acid Molecules H2SO3, H2SO4, and H3PO4, respectively?, model 1 with S=O bonds is incorrect even though this is how we teach highschool and college chemistry.

H2SO3 _____H+ + HSO3-When the acid is heated in the test tube at 150 °C, it gets precipitated. 3H2SO3 ____ 2H2SO4 + H2O + S. When H2SO3 mixes with zinc (Zn) and heat on a light flame, zinc hyposulphite is formed. H2SO3 + Zn _____ ZnS2O4 + 2H2O. H2SO3 has bleaching properties, and it reacts as a bleaching agent because of its .
Sulfurous Acid. Sulfurous acid is a corrosive chemical commonly used in industrial processes, especially at concentrations greater than 90 wt%. It can cause corrosion in metallic and nonmetallic materials, making it important to understand its effects on different materials in various applications.
A step-by-step explanation of how to draw the H2SO3 Lewis Structure (Sulfurous acid). When we have an H (or H2) in front of a polyatomic molecule (like CO.H2SO3 is a weak acid that is made up of a sulfur atom bonded to two oxygen atoms. The sulfur atom is bonded to the two oxygen atoms via a single bond and a double bond, which gives the molecule a bent shape. The acid has a sour taste and is corrosive. It is used in many industrial processes, including the manufacture of fertilizers, textiles .SO2 + H2O = H2SO3 is a Synthesis reaction where one mole of Sulfur Dioxide [SO 2] and one mole of Water [H 2 O] combine to form one mole of Sulfurous Acid [H 2 SO 3] Show Chemical Structure Image. SO2 gas into cup distilled water and test solution obtained by kneeling purple → Seeing purple litmus turns red. The resulting solution is sulfur .
h2so3 h2so3 state of matter It is known that H2SO3 is thermodynamically unstable. In this study, however, we show that a Ci-symmetric dimer of sulfurous acid (H2SO3)2 is 3.5 kcal mol-1 more stable than its dissociation products SO2 and H2O at 77 K. Additionally, we have investigated the kinetic stability of the sulfurous acid monomer with respect to .
H2SO3 đọc là gì? được VnDoc biên soạn hướng dẫn bạn đọc gọi tên axit H 2 SO 3, cũng như đưa ra các nội dung câu hỏi lý thuyết bài tập có liên quan đến axit H2SO3.Hy vọng thông qua nội dung tài liệu bạn đọc còn biết được H 2 SO 3 là axit mạnh hay yếu. Mời các bạn tham khảo chi tiết nội dung dưới đây.
HSO₃⁻ ⇌ H⁺ + SO₃²⁻. Decomposition: When the solution of sulphurous acid is heated in a sealed tube at 150℃, sulphur gets deposited. 3H₂SO₃ → 2H₂SO₄ + H₂O + S. Reducing properties: It acts as a strong reducing agent. When it reduces a certain substance, sulphurous acid is oxidised to H₂SO₄ and gives nascent hydrogen.
H2SO3 + O2 = H2SO4 is a Synthesis reaction where two moles of aqueous Sulfurous Acid [H 2 SO 3] and one mole of Dioxygen [O 2] gas combine to form two moles of aqueous Sulfuric Acid [H 2 SO 4] Reaction Type. Synthesis. Redox; Net Ionic Equation. 2H2SO3(aq) + O2(g) = 2H2SO4(aq) might be an ionic reaction.
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. Since there is an equal number of each element in the reactants and products of H2SO3 + 2H2O = 2H3O + SO3, the equation is balanced.What is Sulphurous Acid? H 2 SO 3 is a chemical compound with yhe chemical name Sulphurous Acid. Sulphurous acid is also called Sulphur dioxide solution or dihydrogen trioxosulphate or trioxosulphuric acid. It is an intermediate species for producing acid rain from sulphur dioxide (SO 2 ).Sulfurous Acid | H2O3S | CID 1100 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.When sulfur dioxide is dissolved in water, an acidic solution results. This has long been loosely called a sulfurous acid, H 2 SO 3, solution. However, pure anhydrous sulfurous acid has never been isolated or detected, and an .h2so3 Learn about the H2SO3 compound, also known as sulfurous acid. See its molecular bonds in the Lewis structure and discover the properties and uses of sulfurous acid. Updated: 11/21/2023.Sulfurous Acid Definition. Sulfurous Acid is a chemical compound which has a formula H 2 SO 3, and is a weak and unstable acid, formed when sulfur dioxide dissolves in water. I was looking at the chemical structure of HX2SOX4 H X 2 S O X 4. Intuitively, I would have expected this molecule to be square planar in accordance with p2d2 p 2 d 2 or sp2d s p 2 d hybridization, but instead it is shown to be in a tetrahedral geometry consistent with sp3 s p 3 hybridization.h2so3 state of matterSulfurous acid has the formula H2 SO 3 and the molecular weight of 82.075 g/mol. Its CAS number is 7782-99-2. There is no clear evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase.There are 4 easy steps to find the molar mass of H2SO3 based on its chemical formula.
WEB8 de jul. de 2019 · “Mindsight é um tipo de atenção focada que nos permite ver o funcionamento da nossa própria mente.” Dr. Daniel Siegel. Por que praticar a atenção .
h2so3|h2so3 state of matter